Classify the following reactions as precipitation reactions oxidation-reduction reactions or acid-base reactions. Addition reactions result in the synthesis of a new molecule giving them their second name.

Precipitation Reactions Wisc Online Oer
A Insoluble product Precipitation reaction The solid that appears when an insoluble product is formed from mixing two aqueous solutions of ionic compounds is called a precipitate and hence such reactions are also called precipitation reactions.

. It is a chemical reaction in which you mix two solutions of two ionic substances and a solid ionic substance a precipitate forms. This solid is referred to as a precipitate. Precipitation reactions can help determine the presence of various ions in solution.
In a reaction in which one of the products has little to no solubility ability to dissolve in water a solid will form. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement double replacement or metathesis. The insoluble salt that falls out of solution is known as the precipitate hence the reactions name.
What is the formula for the compound that forms between sodium and chlorite ions. Precipitation reactions acid-base reactions gas evolution reactions and oxidation-reduction reactions. Up to 10 cash back Double-replacement reaction.
A reaction that forms a solid or precipitate when two aqueous solutions are mixed Precipitate An insoluble product formed through the reaction of two solutions containing soluble compounds. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement double replacement or metathesis reactions. What is the formula for an ionic compound made of aluminum and oxygen.
Write the complete equation for the reaction. Precipitation Questions Describe the solution formed at the instant water solutions of two ionic compounds are mixed before the reaction takes place. Precipitation reactions are usually double displacement reactions.
Precipitation Reactions and Solubility Rules. A precipitation reaction is a type of chemical reaction in which two soluble salts in aqueous solution combine and one of the products is an insoluble salt called a precipitate. These insoluble salts formed in precipitation reactions are called precipitates.
For example precipitation occurs when a part of the atmosphere saturates itself with water vapour and when the right temperature comes its condenses and precipitates. The two processes which make the air saturated. This is because much like rain falls from the sky the solid that forms will fall out of the solution to.
A w B g C s D l. The precipitate stays in the solution as a barrier or it can also be removed from the liquid by using the process of centrifugation decantation or filtration. What is the name of the compound Li3N.
Precipitation refers to a chemical reaction that occurs in aqueous solution when two ions bond together to form an insoluble salt which is known as the precipitate. Precipitation Reactions and Solubility Rules. Comparing KCl aq and aqeous potassium chlorate reactions with aqueous silver nitrate.
A different model for classifying chemical reactions classifies reactions as. NaClaq AgNO3aq H AgCls NaNO3aq Ionic eq. This salt then precipitates out of solution.
Addition reactions occur when two molecules combine to form a larger one leading to a release of energy. Naaq Cl-aq Agaq NO 3. A precipitation reaction is one in which dissolved substances react to form one or more solid products.
Chemical reactions can be classified as. Describe the final mixture. In writing the chemical equation for a precipitation reaction what abbreviation of the physical state must appear with one of the products.
Describe the reaction that takes place in this mixture. The precipitate may stay in the solution as a suspension fall out of solution on its own or can be separated from the liquid using centrifugation decantation or filtration. Precipitation reactions are best classified as which type of reaction.
12NaCl aqK2H2Sb2O7 aqNa2H2SbO7 s2KCl aq 2 CH3COO2Cu aq NH42C2O4 aqCuC2O4 s2CH3COONH4 aq 34Al s3O2 g2Al2O3 s 42Zn sO2 g2ZnO s. Whats In A Name. The term precipitation reaction can be defined as a chemical reaction occurring in an aqueous solution where two ionic bonds combine resulting in the formation of an insoluble salt.
A precipitation reaction is one in which dissolved substances react to form one or more solid products. The solid that separates is called a precipitate. Synthesis decomposition single-displacement and double- displacement.
Formation of an insoluble compound will sometimes occur when a solution containing a particular cation a positively charged ion is mixed with another solution containing a particular anion a negatively charged ion. A precipitation reaction is a kind of chemical reaction in which two soluble salts in a fluid solution mixes and one of the items is an insoluble salt called a precipitate. Propane burns in air to produce carbon dioxide and water.
A precipitation reaction can occur when two solutions containing different salts are mixed and a cationanion pair in the resulting combined solution forms an insoluble salt. Reaction occurs when one compound reacts and is broken down into different elements or simpler compounds. What is this type of chemical reaction called.
Which of the following is a molecular compound. A precipitation reaction refers to the formation of an insoluble salt when two solutions containing soluble salts are combined. Comparing hot KCl vs potassium chlorate reaction with sugar Gummi Bear The non-reaction of potassium chloride with sucrose is contrasted with the reaction of potassium chlorate.
Sometimes a reaction is a synthesis reaction and also a redox reaction.
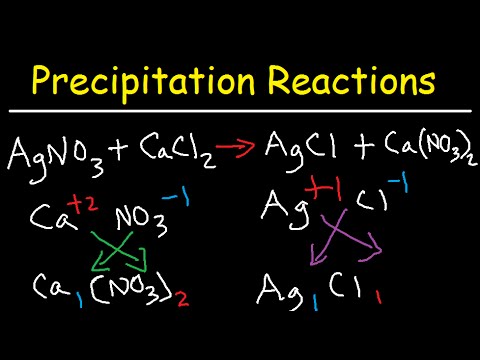
Precipitation Reactions And Net Ionic Equations Chemistry Youtube

Precipitation Reaction A Reaction That Results In The Formation Of An Insoluble Product A Teaching Chemistry Chemistry Education Chemical Equations Chemistry
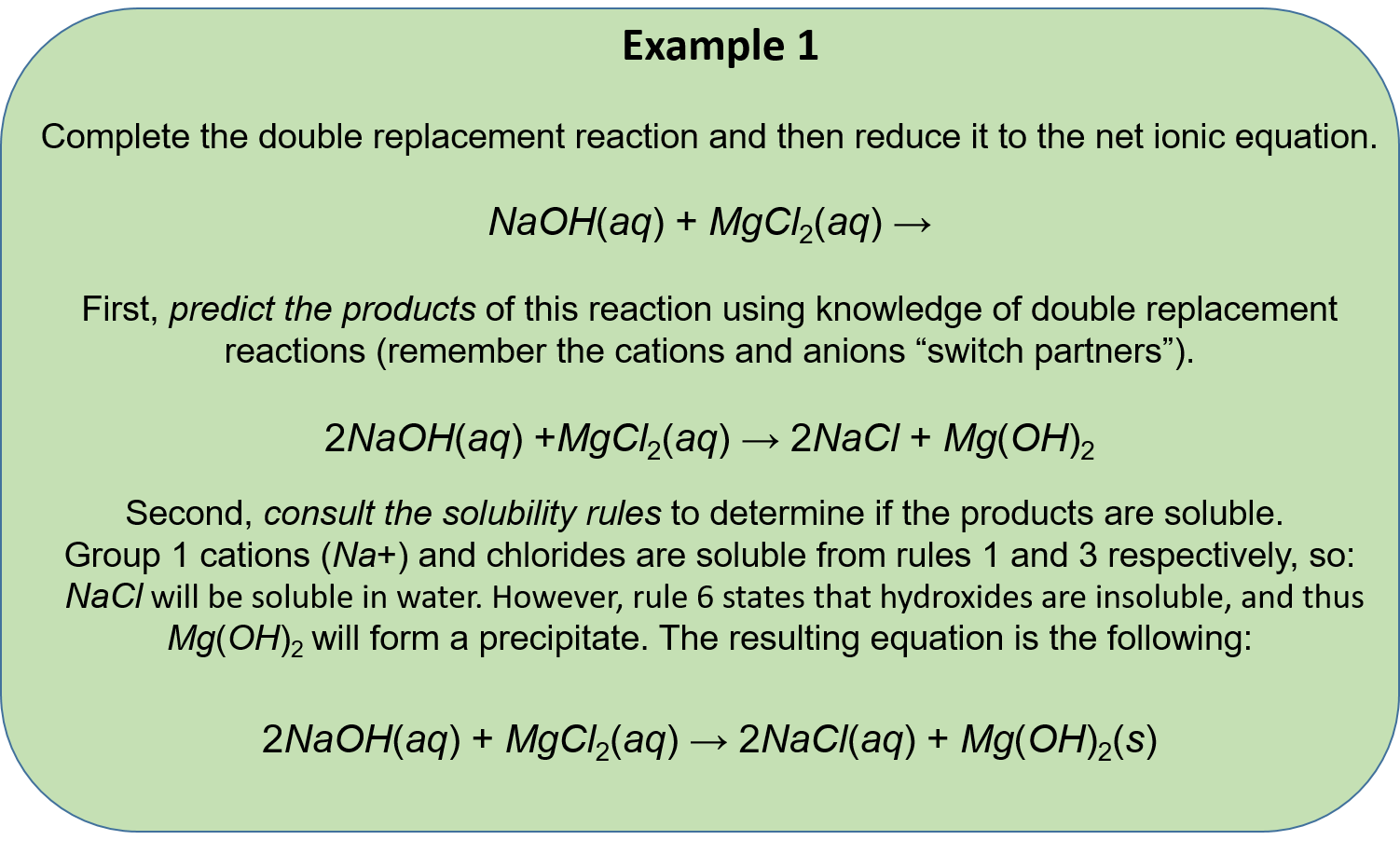
Ch104 Chapter 5 Chemical Reactions Chemistry

Types Of Chemical Reactions Chemistry Education Chemical Reactions Chemistry Class
0 Comments